Industry Leading Platform Enables Process Development and cGMP Manufacturing of Viral Vector Drug Product under 6 Months
We offer a purpose-built facility specifically designed for Viral Vector manufacturing. Our processes adhere to stringent global regulatory guidelines, including FDA, EU, and NMPA standards. Additionally, our experienced team boasts over 20 years in development and GMP manufacturing of gene therapies.

Process Development Scientists and Manufacturing Operators collaborate throughout the development and manufacturing process to ensure successful production.
* Scalability to 500L while consistently maintaining product quality, and productivity from PD to Pilot scale to GMP. CoJourney has successfully executed multiple runs at the 500L scale.


Partnering with you through every step of the development process, from benchtop optimization to full-scale GMP manufacturing and finishing.
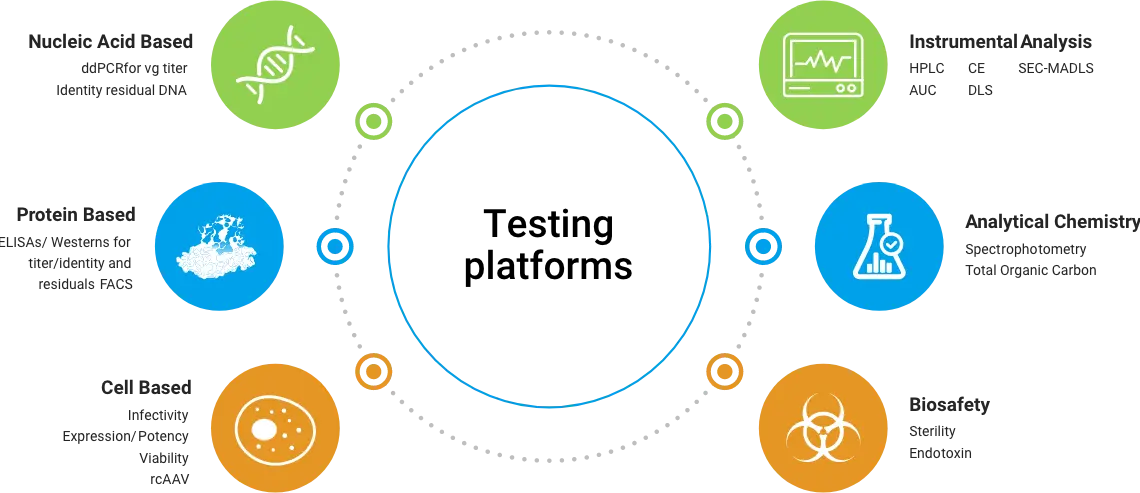
CoJourney’s viral vector platform is specifically designed to maximize yields, increase scalability, and produce viral vectors of the highest quality. Our industry leading, serum free suspension platform delivers upstream yields of up to 1×1012 viral genomes per ml (vg/ml), utilizing a HEK293 triple plasmid co-transfection process. Programs employing this process have obtained IND approval from both the FDA and NMPA. Please download our brochure for more information about our plasmid, viral, vector and testing services.